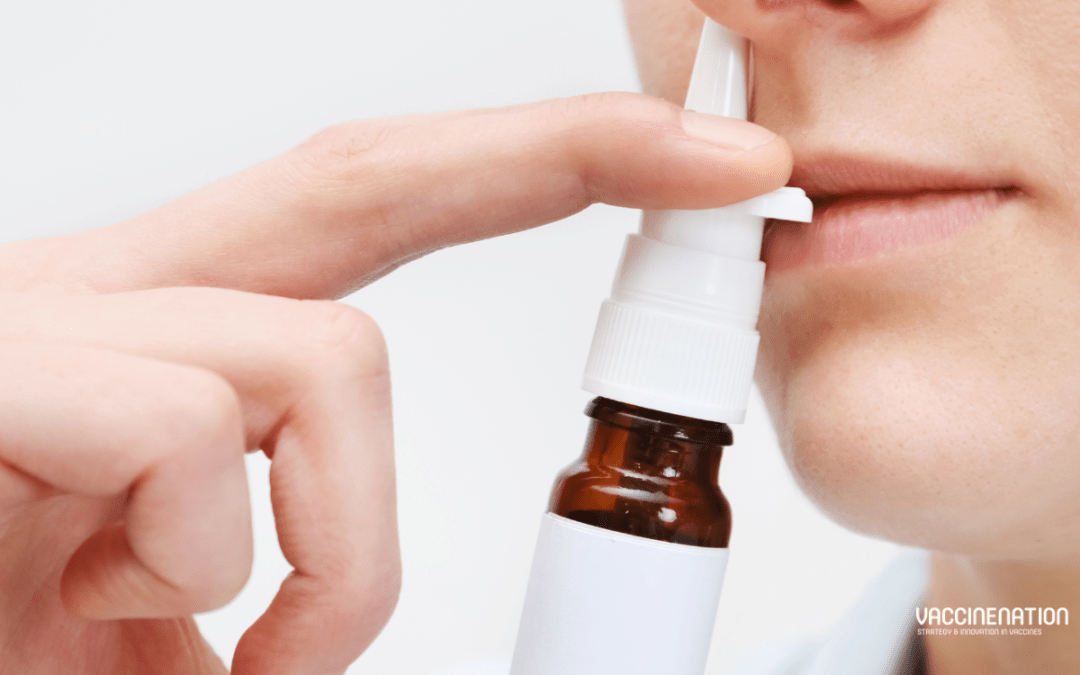
In September 2024 the United States FDA announced the approval of FluMist for self- or caregiver-administration. It is approved for active immunisation for the prevention of influenza disease caused by subtypes A and B in individuals aged 2 to 49. FluMist is administered as a nasal spray and was initially approved for use in 2003, with an expansion to include younger children in 2007. The latest approval was based on a submission that included results from a usability study, which demonstrated that individuals over the age of 18 could self-administer or administer FluMist to eligible recipients. It is the first vaccine to prevent influenza that does not require administration by a health care provider.
FluMist
The live attenuated influenza vaccine (LAIV) is administered as a nasal spray. The vaccine is recommended by the Advisory Committee on Immunisation Practices (ACIP) and American Academy of Paediatrics (AAP). A prescription is required to receive FluMist, but the vaccine can either be administered by a health care provider in a health care setting or by the vaccine recipient or caregiver over 18. Since its initial approval in the United States in 2003, almost 200 million doses have been distributed globally.
In FDA-required studies, AstraZeneca evaluated if individuals from 18 to 49 years of age could “appropriately” administer FluMist when given instructions. These studies showed that 100% of intended users administered a full dose, and that the efficacy, immunogenicity, and adverse events with self-administration were “similar to” those seen with administration in a health care setting.
The vaccine manufacturer will make the vaccine available through a third-party online pharmacy for people who are interested in self- or caregiver-administration. To order FluMist, these people will complete a screening and eligibility assessment before receiving a prescription and delivery of the vaccine. Individuals aged 2 to 17 should not self-administer.
Broader accessibility
Director of the FDA’s Centre for Biologics Evaluation and Research Dr Peter Marks commented that the approval provides a “new option” for safe and effective seasonal influenza vaccination, “potentially with greater convenience, flexibility, and accessibility for individuals and families”.
“Getting vaccinated each year is the best way to prevent influenza, which causes illness in a substantial proportion of the U.S. population every year and may result in serious complications, including hospitalisation and death. This approval adds another option for vaccination against influenza disease and demonstrates the FDA’s commitment to advancing public health.”
Dr Ravi Jhaveri, Division Head, Infectious Disease, Virginia H. Rogers Professor in Infectious Diseases, Professor of Paediatrics, Northwestern University School of Medicine, also emphasised the importance of increasing accessing to vaccinations to address the “significant burden” of influenza.
“For the first time, families and caregivers will be able to protect themselves against influenza with a needle-free, self-administered vaccine, from the convenience of their own home.”
Iskra Reic, Executive Vice President, Vaccines and Immune Therapies, AstraZeneca, stated that the approval of FluMist for self-administration is an “important step forward in making vaccines more accessible to fight the high annual burden of influenza”.
“For more than 20 years, FluMist has been the only nasal spray flu vaccine licensed in the US and now it is also the only vaccine to help individuals, families, and communities access an influenza vaccine conveniently through self- and caregiver-administration outside of traditional healthcare settings.”
To learn more about the various applications of nasal spray vaccines at the Congress in Barcelona next month, get your tickets to join us here. Don’t forget to subscribe to our weekly newsletter for more vaccine insights.